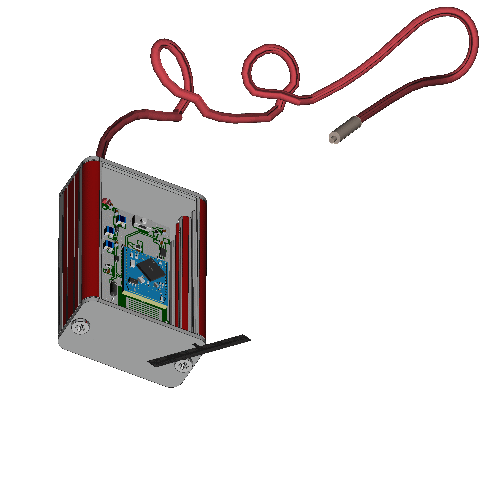
Application
Wireless reference implant hardware for safety testing in MRI.
Contributors
Berk Silemek¹, Frank Seifert¹, Lukas Winter¹
Estimated cost
Progress
CERN-OHL-W v2
Wireless reference implant hardware for safety testing in MRI. Small rms sensors embedded in active implantable medical devices (AIMD) can provide important safety relevant information that can be exploited e.g. by parallel transmission based MR techniques for improved measurement based, subject specific RF safety of implants in MRI. Such concepts require the AIMD to communicate wirelessly with the pTx-capable MR scanner. Here Bluetooth low energy (BLE)-based wireless implant hardware and a communication workflow are presented to perform a variaty of implant safety related investigations.
Specifications
Dimensions:
- Electronics box: 60 x 45 x 25 mm^3
- Lead trajectory lengths: 600 mm for realistic trajectories, 500 mm for the extension wire
- Wire diameter: 2 mm
Connectivity & Measurement Capability:
- Bluetooth v5.2
- 7.8 μs sampling rate (5.3μs ADC averaging)
- 7200 samples / 58 ms memory
- Calibrated linear RMS E-field measurement range: 0.06-1.6 V tested @123MHz (3T) and @297MHz (7T)
Other:
- 110 mAh battery capacity
- Trigger arm time 100 ms
Publications
Affiliations
1Physikalisch-Technische Bundesanstalt (PTB), Berlin, Germany